Compound Management
Connecting and accelerating your drug discovery: rapid compound sourcing and aggregation, secure storage, flexible cherry picking and plating, with full visibility of your inventory at all times
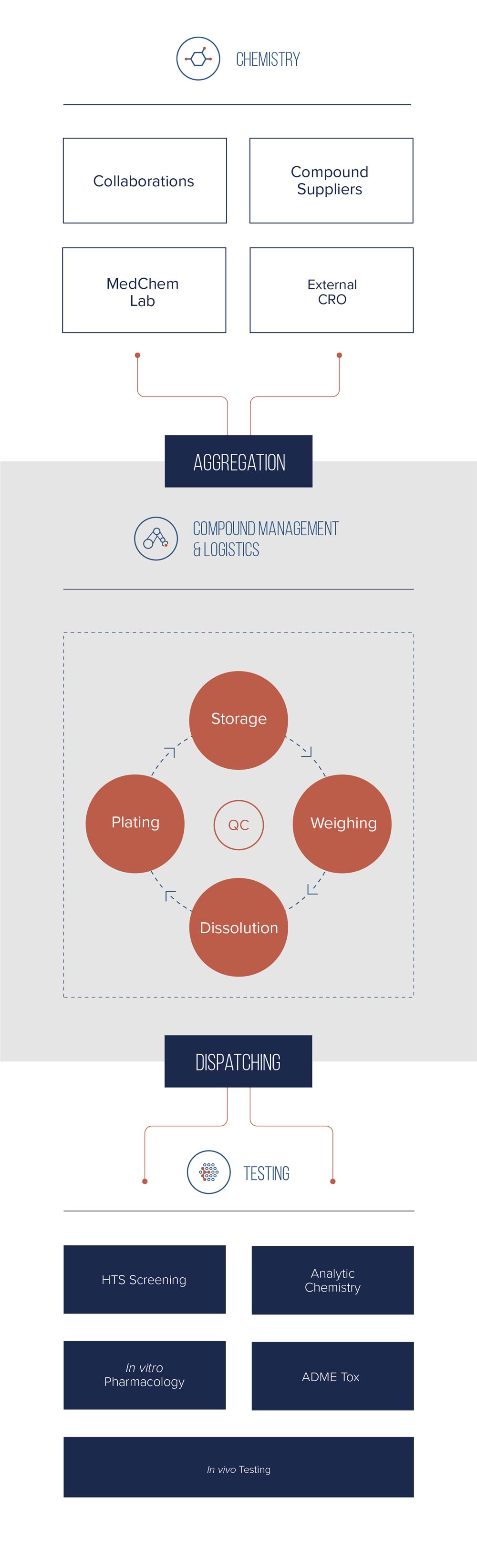
BioAscent offers customers straightforward and cost-effective access to the big-pharma calibre compound management capabilities and expertise at our €20 million facility. We give customers the peace-of-mind that their compounds are aggregated and stored securely, their inventory is up-to-date and trackable, and compounds can be accessed and delivered quickly and easily – something that is especially critical for innovative biotechs working with a network of CROs.

We manage libraries of all sizes, ranging from tens to hundreds-of-thousands of compounds. But our customers all have this in common – they choose BioAscent to cost-effectively and securely manage their compounds, connect their CRO network, and accelerate their design-make-test cycles.
Our customers benefit from rapid compound sourcing and aggregation, secure storage, up-to-date visibility of all samples and orders with a full audit trail, and rapid delivery of solids or liquids in any format, up to and including screen-ready Assay Ready Plates (ARPs).

“ODD has been working with BioAscent for over two years, since we transferred our entire compound management to them. The logistical services are reasonably priced and reduce the burden on our chemists for weighing and dispatching allowing greater productivity. BioAscent can accommodate varying storage requirements for our compounds that reflect differing stabilities and are able to perform analysis on the samples when needed. The shipping turnaround from BioAscent is always rapid and they can accommodate differing shipping formats and often, last-minute changes in our requirements.” – Oxford Drug Design.
We manage libraries of all sizes for over 70 customers from across the globe, ranging from big pharma to virtual biotechs and charity and academic consortia in both North America and Europe, and we currently hold and manage over 1.5 million compounds for them in both liquid and solid formats. Via the European Lead Factory we also provide compound management for collections from AstraZeneca, Bayer, Grunenthal, Janssen, Merck KgaA, Sanofi, Servier and UCB.
Our expertise in cherry picking, reformatting, shipping and data management has allowed us to deliver over 100,000 screening plates globally to our customers and their partner testing laboratories since 2013.
“VAST Bioscience was looking for a compound management provider that is competitively priced, and is fast and flexible in order to service both our screening collaborations of VAST libraries, and our internal drug discovery programs. We have been very pleased with the professionalism that BioAscent has brought to the table, in particular the rapid response and turnaround time for delivering plating orders and shipment, combined with the flexibility and attention to detail. BioAscent has been reliable, timely and accurate, and it was very pleasing to see the positive impact on our internal and external projects.” – VAST Bioscience
Our compound management services allow our customers to both save costs and accelerate their drug discovery. Operating since 2005, our €20 million facility stores and manages customer libraries ranging in size from tens to hundreds-of-thousands of compounds in both liquid and solid formats. We support all stages of the drug discovery process from screening and hit identification through to lead optimisation and candidate selection, with on-site in vitro biosciences and medicinal chemistry available if required.

BioAscent can support some or all of your key compound management activities including aggregation, storage, weighing, dissolution, plating (including acoustic dispensing and Assay Ready Plates), LC-MS analysis and distribution. We provide both standalone activities on a fee-for service basis, as well as fully integrated compound management services. However you work with us, a dedicated account manager works with you and your R&D partners, and our transparent activity pricing makes it easy to calculate cost savings.
Developed by BioAscent, Compound Connect is our web-based interface providing customers with secure access to a customised, online inventory and ordering system for their compound library. The fully auditable order fulfilment system interfaces with the BioAscent Titian Mosaic compound management system and is also compatible with existing customer informatics systems.
Compound Connect is easy to use and secure, and allows customers to:
“Our partnership with BioAscent combines our long-standing expertise in high content cell based phenotypic screening with BioAscent’s expertise in compound management and quality control processes for compound storage, identification and resupply. Our discussions with BioAscent have been very dynamic and interactive which has helped ensure we developed the compound management solution which best meets our needs. The ongoing relationship with BioAscent and their fast response times are directly supporting our broader goals to establish a world leading cell based screening capability which spans across multiple disease areas” – Neil Carragher, Professor of Drug Discovery, University of Edinburgh.
Operating since 2005, our €20 million facility stores and manages customer libraries ranging in size from tens to hundreds-of-thousands of compounds in both liquid and solid formats. We continually invest in new cutting-edge equipment, software and expertise to provide our customers with cost-effective, big-pharma calibre compound management services, and the cycle-time savings and security that these services deliver.
The BioAscent compound management team is led by Dr Sylviane Boucharens. A drug discovery and compound management expert with over 20 years of pharmaceutical industry experience, Sylviane led the global compound repository team and the in vitro screening team at the Merck Sharpe & Dohme / Schering Plough / Organon site in Newhouse, Scotland at which BioAscent is now based.
Sylviane is supported by a team of experts in compound management, data management, software development, logistics, and computational chemistry working in state-of-the-art facilities originally commissioned to manage Merck Sharpe & Dohme’s global collection.

Associate Director, In Silico Discovery and Data Analysis
Angelo is a highly experienced computational chemist, project leader and strategic contributor to discovery teams, and has over 15 years’ experience working across different organisations in US and UK. He has a PhD in computational chemistry from the University of Nottingham. Prior to joining BioAscent, Angelo led the computational chemistry and artificial intelligence team at the drug discovery unit at the Beatson Institute for Cancer Research in Glasgow. Angelo is an expert in in silico structure-based and fragments-based methodologies applied to drug design with a passion for machine learning. He is a co-author on more than 30 publications and patents including a book chapter on fragments library design. Angelo is also a reviewer for several highly regarded journals and fellowships, and serves as an advisory board member for several international conferences.

Director of Chemistry
Angus has over 15 years’ experience as a practising medicinal chemist leading both project and medicinal chemistry delivery in multinational and multidisciplinary teams. In his most recent role as Head of Chemistry at the European Screening Centre Newhouse he was Medicinal Chemistry lead of the European Lead Factory project responsible for over 30 hit to leads programmes. Prior to this Angus has held leadership roles at Organon, Schering-Plough, MSD, Prosidion and Redx Oncology. During his time in pre-clinical drug discovery he has had broad exposure and experience of working across a diverse range of therapeutic areas and target classes from hit generation to candidate nomination. Angus has a PhD in Organic Chemistry from Strathclyde University and carried out Postgraduate studies at Cambridge University. He is a co-author on over 30 papers and patents.

Director of Chemistry
Angus has over 15 years’ experience as a practising medicinal chemist leading both project and medicinal chemistry delivery in multinational and multidisciplinary teams. In his most recent role as Head of Chemistry at the European Screening Centre Newhouse he was Medicinal Chemistry lead of the European Lead Factory project responsible for over 30 hit to leads programmes. Prior to this Angus has held leadership roles at Organon, Schering-Plough, MSD, Prosidion and Redx Oncology. During his time in pre-clinical drug discovery he has had broad exposure and experience of working across a diverse range of therapeutic areas and target classes from hit generation to candidate nomination. Angus has a PhD in Organic Chemistry from Strathclyde University and carried out Postgraduate studies at Cambridge University. He is a co-author on over 30 papers and patents..

David is a Partner at Maven Capital Partners where he is a member of Maven’s Investment Committee and is responsible for sourcing and executing private equity investments in Scotland as well as technology and life science investments across the UK. His current investments include Blacktrace Group, Curo Compensation, Optoscribe, QikServe, CB Technology and BioAscent. David started his career as a scientist with GlaxoSmithKline.
After completing his PhD, he moved to international consultancy firm Wood Mackenzie (and then parent Deutsche Bank) where he supported the healthcare banking team, advising clients on their corporate and licensing strategies; and performing due diligence projects on companies, products and technologies.
David joined Aberdeen Asset Management’s private equity division (spun-out to form Maven in 2009) in September 2007 as Fund Manager for an early-stage technology fund. David has a First Class Honours degree in Pharmacy, a PhD (Molecular Biology/Gene Delivery) from the University of Bath and a MBA from The University of Edinburgh.

Associate Director of Chemistry
Duncan is an experienced medicinal chemist having worked in the pharmaceutical and not-for-profit sectors for over 18 years. He has broad scientific expertise in several therapeutic areas, working in both lead finding and lead optimisation project teams. Duncan is expert in fragment-based hit finding strategies and developing HTS campaigns. In his previous roles at MSD and more latterly, the Beatson Institute for Cancer Research (BICR), Duncan led multiple internal and cross-site collaborative project teams and managed outsourced chemistry efforts across different companies. Duncan spent over 10 years at the BICR working in the area of fragment-based drug discovery, prioritising and developing mM affinity fragment hits to high affinity lead series. Duncan is a co-author on 20 papers and patents.

CEO BioCity
Glenn Crocker is an experienced CEO, company founder, non-executive director and investor in the life sciences sector. He has been CEO of BioCity Group since mid-2003, shortly after it was founded. He has a DPhil in Immunology from Oxford University and qualified as a chartered accountant with EY, focussing on working with biotech companies in Palo Alto, California and Cambridge, UK, where he headed up the UK Biotech practice for EY.
BioCity builds, funds and grows life science companies at its four incubator sites in the UK.
Glenn is a non-executive director of a number of companies and consults on business incubation, start-up creation and cluster growth. He has been directly involved in investing in life science companies through BioCity’s own or managed funds since 2006.
In 2014 Glenn received an MBE for services to the biotechnology industry.

Dr Louis J. Nisbet D.Sc. - Vivanova Ltd
Louis began his career in pharmaceuticals R&D with Roche, Glaxo and SmithKline during which period he achieved international recognition in the discovery of NCEs in infectious diseases. He was one of the UK’s first biotechnology entrepreneurs establishing Xenova and Brax both of which achieved successful exits via IPO and trade sale respectively.
He was the first recipient of the Ernst & Young Biotechnology Entrepreneur of the Year Award. Since 2000 he has been active in investing and building businesses in the private and public equity markets across the health sector. Venture capital investments have been with Kurma Partners, Sitka Partners, Noble Group and Alderley Park Ventures.
He has considerable executive and non-executive Board experience with over 20 quoted and private companies. He has a Ph.D. from Imperial College, London and D.Sc. from the U of Strathclyde.

Chief Commercial Officer
Mike is an experienced life sciences professional with a track record in sales and marketing strategy, general management, licensing and contract negotiation. Mike was formerly Head of Sales and Marketing at CXR Biosciences, an investigative toxicology CRO. Following CXR’s acquisition by Concept Life Sciences in 2015 he became Vice President Business Development Safety & Toxicology. Prior to his time at CXR, Mike worked in business development and licensing at the specialty pharmaceutical company Ardana plc, and held various pharmaceutical industry management consultancy roles at ZS Associates, Deloitte and Arthur Andersen Business Consulting. Mike has a PhD in genomics from the University of Cambridge, MRC Laboratory of Molecular Biology.

Chief Scientific Advisor
Phil has over 30 years medicinal chemistry and drug discovery experience from Roche, Organon, Schering-Plough, Merck and the University of Dundee including senior roles as Executive Director and Acting Site Head. He was a member of the Research Leadership Council at Schering-Plough. Numerous clinical candidates resulted from groups for which he was responsible. These span a broad range of target families and therapeutic areas. He has led the European Screening Centre team at Biocity Scotland, Newhouse for the past 5 years and this group plays a key role in delivering validated hit series for SMEs and academics through the IMI funded European Lead Factory. Phil’s postgraduate studies were carried out at the University of Manchester and Imperial College. Phil was elected a Fellow of the Royal Society of Chemistry in 2005, he chaired the Medicinal Chemistry interest group of the Royal Society of Chemistry for 8 years, chaired the organising committee of the European Federation of Medicinal Chemistry – International Symposium on Medicinal Chemistry (EFMC-ISMC) in 2016 and sat on the Executive Committee of the EFMC for four years. He is a co-author on over 70 scientific papers and patents.

Chief Scientific Advisor
Phil has over 30 years medicinal chemistry and drug discovery experience from Roche, Organon, Schering-Plough, Merck and the University of Dundee including senior roles as Executive Director and Acting Site Head. He was a member of the Research Leadership Council at Schering-Plough. Numerous clinical candidates resulted from groups for which he was responsible. These span a broad range of target families and therapeutic areas. He has led the European Screening Centre team at Biocity Scotland, Newhouse for the past 5 years and this group plays a key role in delivering validated hit series for SMEs and academics through the IMI funded European Lead Factory. Phil’s postgraduate studies were carried out at the University of Manchester and Imperial College. Phil was elected a Fellow of the Royal Society of Chemistry in 2005, he chaired the Medicinal Chemistry interest group of the Royal Society of Chemistry for 8 years, chaired the organising committee of the European Federation of Medicinal Chemistry – International Symposium on Medicinal Chemistry (EFMC-ISMC) in 2016 and sat on the Executive Committee of the EFMC for four years. He is a co-author on over 70 scientific papers and patents.

Director of Biosciences
With over 15 years working in drug discovery Stuart has extensive experience of developing and trouble-shooting novel screening assays, designing screening cascades, compound screening, hit validation and supporting hit to lead and lead optimisation programs. Throughout the 5 years of the European Lead Factory project he held the position of Head of Biology at the European Screening Centre (ESC), leading a team of bioscientists in prosecuting and triaging the output of over 90 high throughput screens across all major target classes and disease indications. Prior to this Stuart helped establish the Dundee Drug Discovery Unit (DDU) where he spent 7 years as a project and then team leader working across a wide array of novel drug targets and assays. He also had a leading operational role in DDU-Industry partnerships with both large pharma and SMEs.

Director of Biosciences
With over 15 years working in drug discovery Stuart has extensive experience of developing and trouble-shooting novel screening assays, designing screening cascades, compound screening, hit validation and supporting hit to lead and lead optimisation programs. Throughout the 5 years of the European Lead Factory project he held the position of Head of Biology at the European Screening Centre (ESC), leading a team of bioscientists in prosecuting and triaging the output of over 90 high throughput screens across all major target classes and disease indications. Prior to this Stuart helped establish the Dundee Drug Discovery Unit (DDU) where he spent 7 years as a project and then team leader working across a wide array of novel drug targets and assays. He also had a leading operational role in DDU-Industry partnerships with both large pharma and SMEs.

Chief Operating Officer and Co-founder
Sylviane has more than 20 years’ experience in scientific research and drug discovery environment. Sylviane has a track record in building and leading outstanding and productive teams into seamless successful enterprises in start-up and medium and large pharma environment. Prior to BioAscent Sylviane was Head of Global Compound repository and in-vitro Screening (Merck Sharp & Dohme, Newhouse Scotland). She was also responsible for managing a portfolio of early Drug Discovery projects in Cardiovascular from Target Identification and validation to Lead Optimisation. Sylviane attained her PhD in Cellular and Molecular Biology from the University Joseph Fourier, Grenoble, France, followed by post-doctoral positions at the INSERM U366 Laboratory, France and University of Manchester, UK under the Wellcome Trust International Fellowship.

Chief Operating Officer and Co-founder
Sylviane has more than 20 years’ experience in scientific research and drug discovery environment. Sylviane has a track record in building and leading outstanding and productive teams into seamless successful enterprises in start-up and medium and large pharma environment. Prior to BioAscent Sylviane was Head of Global Compound repository and in-vitro Screening (Merck Sharp & Dohme, Newhouse Scotland). She was also responsible for managing a portfolio of early Drug Discovery projects in Cardiovascular from Target Identification and validation to Lead Optimisation. Sylviane attained her PhD in Cellular and Molecular Biology from the University Joseph Fourier, Grenoble, France, followed by post-doctoral positions at the INSERM U366 Laboratory, France and University of Manchester, UK under the Wellcome Trust International Fellowship.

Chief Operating Officer and Co-founder
Sylviane has more than 20 years’ experience in scientific research and drug discovery environment. Sylviane has a track record in building and leading outstanding and productive teams into seamless successful enterprises in start-up and medium and large pharma environment. Prior to BioAscent Sylviane was Head of Global Compound repository and in-vitro Screening (Merck Sharp & Dohme, Newhouse Scotland). She was also responsible for managing a portfolio of early Drug Discovery projects in Cardiovascular from Target Identification and validation to Lead Optimisation. Sylviane attained her PhD in Cellular and Molecular Biology from the University Joseph Fourier, Grenoble, France, followed by post-doctoral positions at the INSERM U366 Laboratory, France and University of Manchester, UK under the Wellcome Trust International Fellowship.

Ian is a Chartered Accountant and an experienced Finance Director/Chief Financial Officer with a successful track record gained in SMEs and PLCs in the manufacturing and customer service industries - particularly within life sciences, pharmaceuticals and FMCG.
Ian has successful record of achievement as a CFO within Private Equity backed businesses with credibility gained from developing positive and effective relationships with stakeholders, and successful participation in exits with a proven track record in securing funding for growth from both public sector finance providers as well as institutional finance providers.
Ian’s career is varied having worked primarily in the UK but with extensive overseas travel. As well as heading up a number of large finance teams Ian’s experience extends to successfully evaluating and commercially shaping high value projects including worldwide ERP system implementations as well as mergers and acquisition experience.

Chief Executive Officer
An experienced Senior Executive with both General Management and Business Development experience in the pharmaceutical/CRO arena. Paul has an understanding of the drug development process from discovery to clinical research gained through more than 29 years in the CRO/pharmaceutical sector. A strategic thinker and management professional with experience gained in both small and large companies and with recent start-up experience including leading funding discussions with VCs and other corporate finance groups. Paul recently turned around and sold CXR Biosciences to Concept Lifesciences in late 2015. Elected Fellow of the Royal Society of Biology, 2013.

Chief Executive Officer
An experienced Senior Executive with both General Management and Business Development experience in the pharmaceutical/CRO arena. Paul has an understanding of the drug development process from discovery to clinical research gained through more than 29 years in the CRO/pharmaceutical sector. A strategic thinker and management professional with experience gained in both small and large companies and with recent start-up experience including leading funding discussions with VCs and other corporate finance groups. Paul recently turned around and sold CXR Biosciences to Concept Lifesciences in late 2015. Elected Fellow of the Royal Society of Biology, 2013.